MODEL AContact us
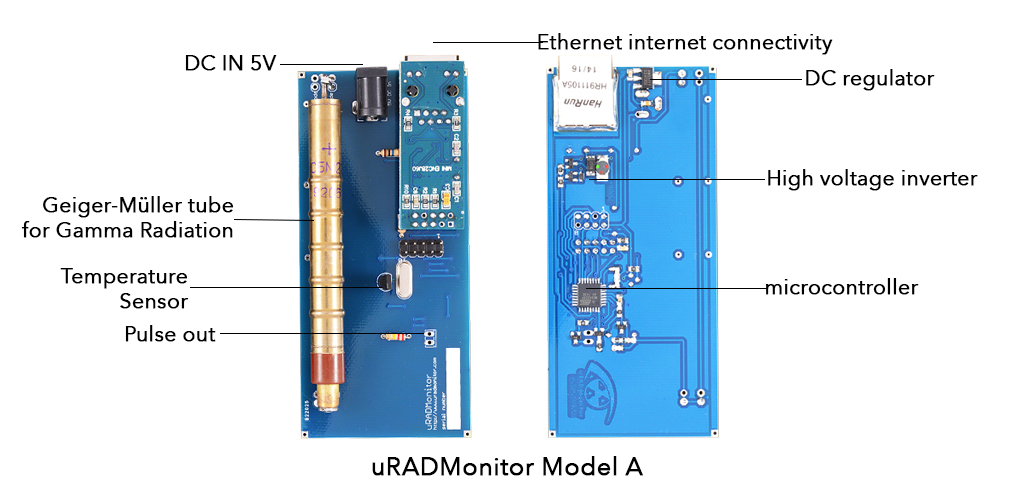
A high quality, automated radiation dosimeter, in a rugged rainproof aluminium enclosure. It runs on little power and doesn't need a computer. Measurements are transmitted automatically to the Internet using an Ethernet connection to your internet Router. They can be accessed on the uRADMonitor portal or in your local network, directly.
George, Ukraine: "The first impression to me was like "beauty!" :) I like the idea of global radiation monitoring very much not only because it is a cool idea itself, but maybe because some time ago our country severelly suffered from Chernobyl disaster(most of Chernobyl's radiactive residues were spilt across our country's territory)"
| Sensor | Parameter | Minimum value | Maximum value |
| Dallas DS18B20 | Temperature | -55 °C | +125 °C |
| SBM20 * | γ,x-rays | 0.01μSv/h | 9999.99μSv/h |
[1] Radiation Health Effects, US Environmental Protection Agency
| Item | Parameter | Ratings |
| Voltage | External | 5V - 9V |
| Connectivity | Ethernet | |
| Microcontroller | Atmega328p | 8 bit |
| Enclosure | Rugged aluminium | 110x80x24 mm |
| Temperature | Operating Range | -20°C to +65°C |
MODEL KIT1Contact us
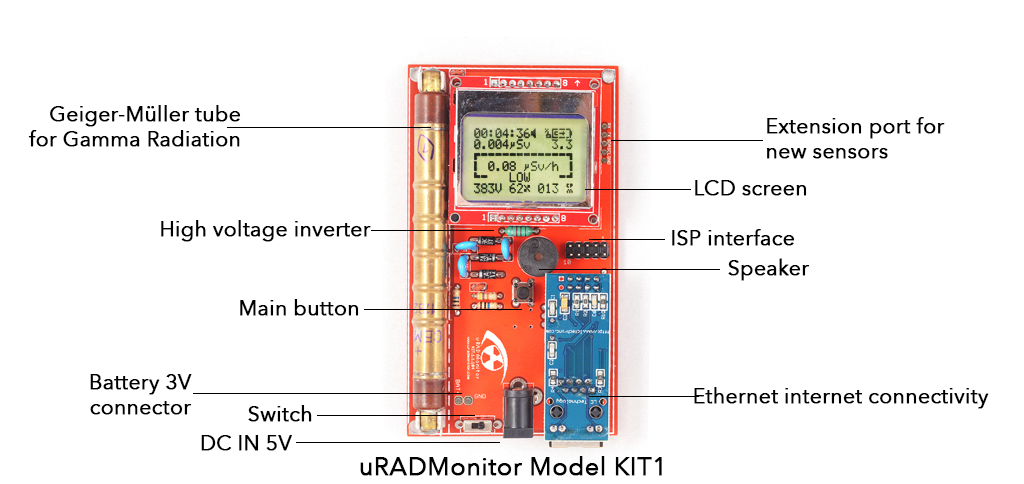
Intended as open source for those who want to build their own dosimeter with their own tools, this is also available pre-assembled. It measures Gamma radiation and with the integrated Ethernet connectivity will send all measurements automatically via the Internet. Add a battery and it can also be used as a portable dosimeter, showing all measurements on the LCD.
Andy, USA: "Many thanks for such great products."
| Sensor | Parameter | Minimum value | Maximum value |
| SBM20 | γ,x-rays | 0.01μSv/h | 9999.99μSv/h |
| Hackable | Add any additional sensors |
[1] Radiation Health Effects, US Environmental Protection Agency
| Item | Parameter | Ratings |
| Voltage | External | 5V - 9V |
| Battery | 3V (2xAA) | |
| Connectivity | Ethernet | |
| Microcontroller | Atmega328 | 8 bit |
| Size | 110x60x12 mm |
MODEL DContact us
This is a compact portable environmental dosimeter, capable of measuring Alpha, Beta and Gamma radiation but also air quality as PM2.5 and Volatile organic compounds. It is a modern design, featuring a large colour LCD screen with touchscreen, a GPS receiver to tag all measurements and a SDCard slot. It comes with WLAN connectivity to send all data online.
Urs, Switzerland: "My second A3 has just been delivered - perfect service!"
| Sensor | Parameter | Minimum value | Maximum value |
| Bosch BME680 | Temperature | -40 °C | +85 °C |
| Pressure | 300 hPa | 1100 hPa | |
| Humidity | 0% RH | 100% RH | |
| VOC | 0 mg/m³ | 100 mg/m³ reducers 10 mg/m³ oxidizers | |
| Sharp GP2Y1010AU0F | PM2.5 | 0 μg/m³ | 800 μg/m³ |
| LND LND712 * | α,β,γ,x-rays | 0.005μSv/h | 5000.00μSv/h |

Ionizing radiation is harmful to living organisms because it can cause damage to cells that can result in multiple disorders , the most common of which is cancer. Ionizing radiation is naturally occurring from cosmic and terrestrial sources, but there are also artificial generators related to nuclear activities or x-ray devices . Worldwide global average dose is 3.01mSv [2].
Particulate matter PM2.5 refers to small particles with a diameter of up to 2.5 microns. These particles can penetrate deep into the lungs , causing allergies, respiratory and cardiovascular diseases [3].
[1] Volatile Organic Compounds' Impact on Indoor Air Quality, US Environmental Protection Agency
[2] Radiation Health Effects, US Environmental Protection Agency
[3] Health and Environmental Effects of Particulate Matter (PM), US Environmental Protection Agency
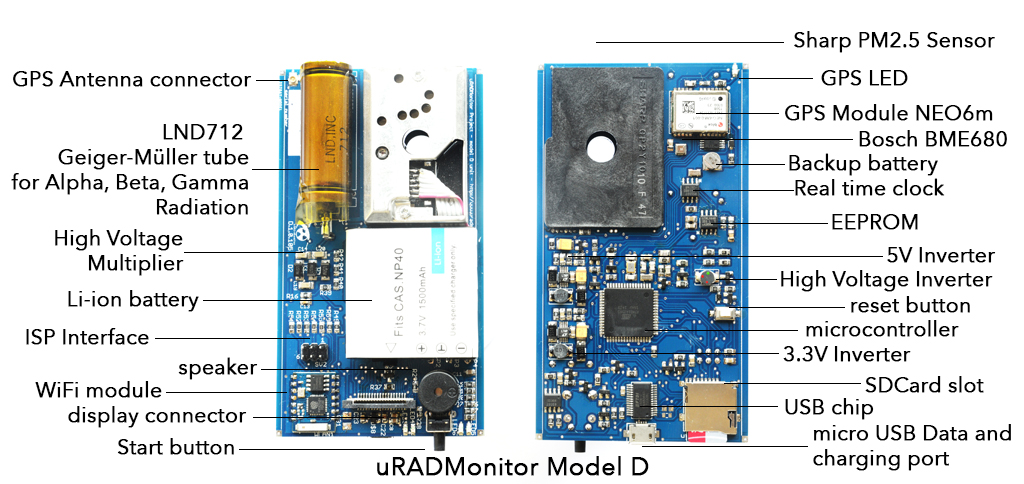
| Module | Parameter | Ratings |
| Battery | Lithium Ion | 1500mAh |
| GPS | Channels | 50 |
| First fix | 27s / 1s | |
| Accuracy | 2m | |
| LCD | Size | 2.4" |
| Touchscreen | resistive | - |
| Wifi | Protocols | 802.11b/g/n |
| SDCard | Type | MicroSD SDHC |
| Microcontroller | Atmega2561 | 8 bit |
| Real Time Clock | GPS synced | - |
| Enclosure | Rugged aluminium | 110x70x24 mm |
Technical Datasheet
API and Server Specs
Product Limited Warranty terms
uRADMonitor with WIFI Configuration manual
USB Driver
In their recent versions, Windows, Linux and MacOS come with built in drivers for your uRADMonitor unit. Use the following resources only if the built in drivers do not work for you.
CH340g driver for Windows
CH340g driver for Linux
CH340g driver for MacOS
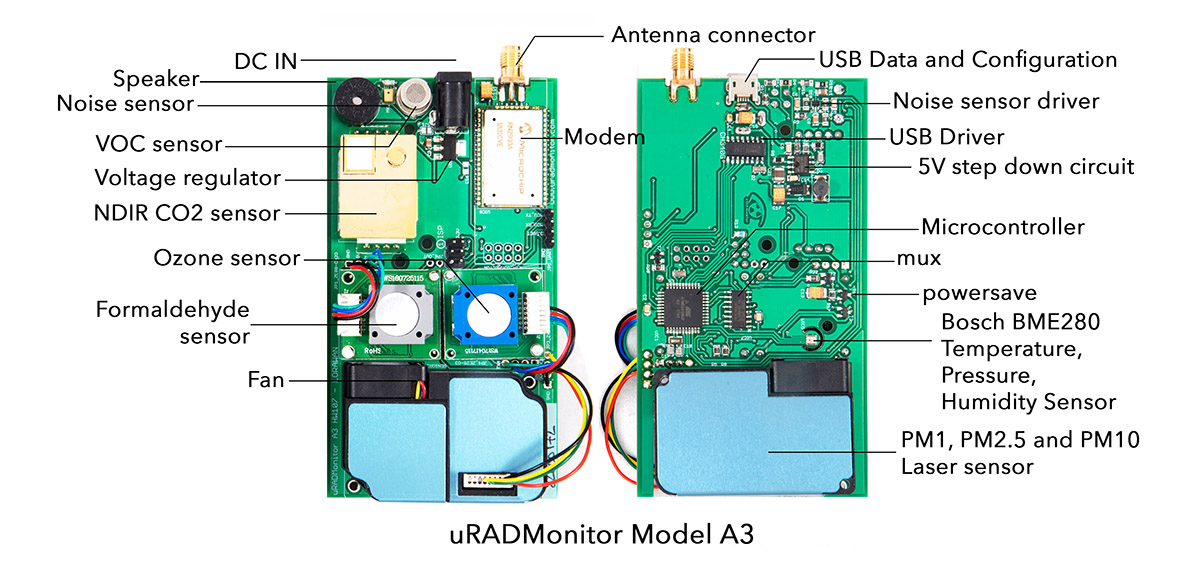
MODEL A3Contact us
Plug and play advanced air quality monitoring station, enclosed in an aluminium body for rugged design, it has sensors for Particulate Matter (PM2.5, PM1, PM10), Ozone, Formaldehyde, Carbon Dioxide, Volatile Organic Compounds (VOC), temperature, barometric pressure, air humidity and noise. The A3 comes in 4 variants with connectivity options: Ethernet, Wifi, GSM (with a data sim card) and LoraWAN. This monitor is lab tested for data accuracy.
Steve, Canada: "Your design is beautiful and the concept is brilliant. It's somewhat surprising that it hasn't been done before (by civilians). All survey meters should be connected to the net. Maybe in the next generation of smart appliances."
| Sensor | Parameter | Minimum value | Maximum value |
| MEMs | Temperature | -40 °C | +85 °C |
| Humidity | 0% RH | 100% RH | |
| Laser scattering | PM1.0 | 0 μg/m³ | 1000 μg/m³ |
| PM2.5 | 0 μg/m³ | 1000 μg/m³ | |
| PM10 | 0 μg/m³ | 1000 μg/m³ | |
| Electrochemical | Formaldehyde | 0 ppm | 5 ppm |
| Electrochemical | Ozone | 0 ppm | 10 ppm |
| NDIR | Carbon Dioxide | 400 ppm | 5000 ppm |
| MOX | VOC | 10 ppm | 1000 ppm * |
| Analogue sound sensor | Noise level | 30dB | 130dB |
To see complete specs see the technical datasheet.

Particulate matter PM2.5 refers to small particles with a diameter of up to 2.5 microns. These particles can penetrate deep into the lungs , causing allergies, respiratory and cardiovascular diseases [2]
Formaldehyde is a toxic colorless gas with a pungent smell, that results from the burning of carbon based materials. It can be found in forest fires, in automobile exhaust and cigarette smoke. It is an allergenic and a known carcinogenic compound that can cause serious health effects, depending on concentration and exposure. Even in tiny quantities just above 0.1ppm it can irritate the eyes and nose, and can worsen asthma symptoms [3]
Carbon dioxide is a gas heavier than air. In small quantities of up to 5000ppm (0.5% ) can cause headaches, lethargy, slowing of intellectual ability, irritability, sleep disturbance. In larger quantities can cause dizziness, loss of sight, hearing or knowledge. The fresh air contains between 360ppm and 410 ppm of CO2 [4]
Ozone can cause the muscles in the airways to constrict, trapping air in the alveoli. This leads to wheezing and shortness of breath. Long-term exposure to ozone is linked to aggravation of asthma, and is likely to be one of many causes of asthma development. Long-term exposures to higher concentrations of ozone may also be linked to permanent lung damage, such as abnormal lung development in children. [5]
Noise Induced Hearing Loss (NIHL) is the most common and often discussed health effect, but research has shown that exposure to constant or high levels of noise can cause countless adverse health affects. [6]
[1] Volatile Organic Compounds’ Impact on Indoor Air Quality, US Environmental Protection Agency
[2] Health and Environmental Effects of Particulate Matter (PM), US Environmental Protection Agency
[3] ToxFAQs™ for Formaldehyde, Agency for Toxic Substances and Disease Registry
[4] Effects of low-level inhalation exposure to carbon dioxide in indoor environments
[5] Health Effects of Ozone Pollution, US Environmental Protection Agency
[6] Noise Pollution, US Environmental Protection Agency
| Item | Parameter | Ratings |
| Voltage | External | 6V - 28V |
| Connectivity | 4 options | Ethernet, WiFi, GSM, LoRaWAN |
| Microcontroller | Atmega1284p | 8 bit |
| Enclosure | Rugged aluminium | 110x80x24 mm |
If you have a radio variant with an antenna, connect the antenna first. If it is the wired variant, connect it to the Internet Router using the Ethernet cable. Connect the uRADMonitor to the power source using a DC adapter with voltage between 6V and 28V or via the USB port with a 5V USB adapter.
The Ethernet variant
Make sure the device is connected to the Internet router using the Ethernet cable. Use a longer cable if needed. The Internet router must have DHCP enabled. When powered, the uRADMonitor gets an IP automatically, via DHCP, and will show up on the map automatically.
The WIFI variant
Use a smartphone or a computer with WLAN capabilities to connect to the local hotspot spawned by your A3 unit. The SSID is uRADMonitor-XX, where XX are the last two digits of the Device ID number. The key is the Device ID, in uppercase, as printed on the enclosure. Open 192.168.4.1 in your browser, and click the "WIFI" link to setup the connection to the Internet AP. Enter the SSID and key of your Internet Access Point. If the connection fails, you will see the status message, and three consecutive beeps will indicated the problem. Alternatively you can change the configuration using the USB data port. See the USB manual.
The LORAWAN variant
Your device must be pre-provisioned with the LoraWAN Gateway and network server details. Alternativelly you can change the configuration using the USB data port. See the USB manual.
The GSM variant
A data SIM card must be inserted in your A3 unit before it can connect over Internet.
Starting with hardware version HW106, the LoRaWAN, Wifi and GSM settings can be customized via the USB connection. Consult your product manual for complete details.
Product Manual
Technical Datasheet
USB Serial Commands Manual
LoRaWAN Data Server Callback manual
Product Limited Warranty terms
uRADMonitor with WIFI Configuration manual
Data access:
Terms of Service
API Terms of Service
API and Server Specs
Direct Data access
Payload Structure (FW78)
Accuracy lab tests and certifications
INCD ECOIND Report (ISO 17025 compliant), for testing the A3 against the reference methods
AQMD AQ-SPEC Performance test report
USB Driver
In their recent versions, Windows, Linux and MacOS come with built in drivers for your uRADMonitor unit. Use the following resources only if the built in drivers do not work for you.
USB Driver for Windows
USB Driver for Linux
USB Driver for MacOS
RO:
RO: Ghid rapid de utilizare
Fisa tehnica
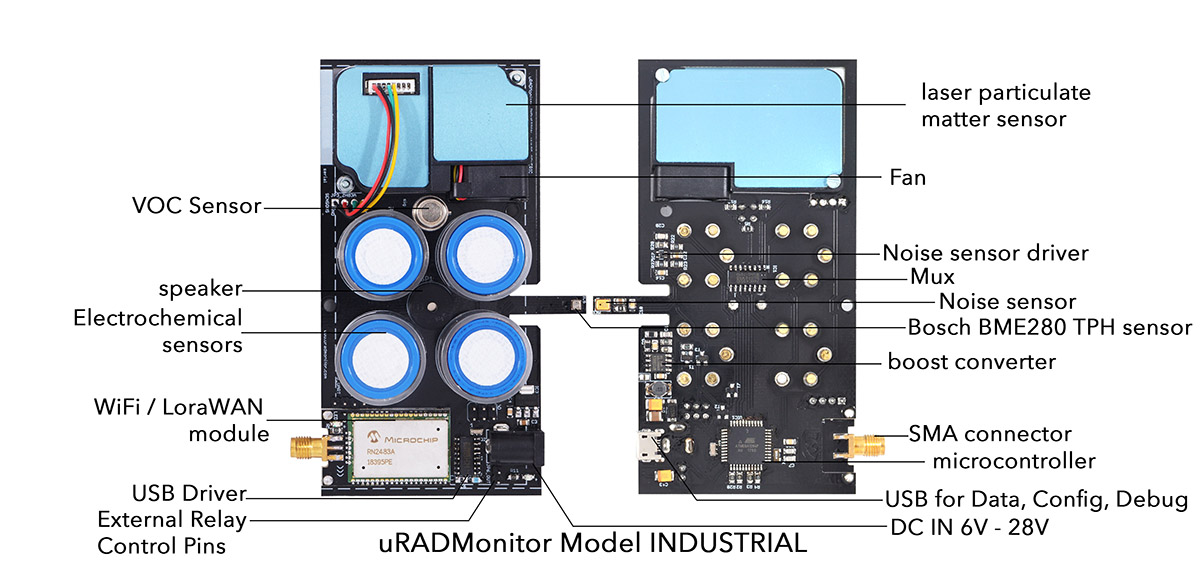
MODEL INDUSTRIALContact us
An automated, fixed monitoring station that tracks a total of 11 important air quality parameters including Particulate Matter, Carbon Monoxide, Ozone, Sulphur Dioxide, Nitrogen Dioxide and more for the INDUSTRIAL sector. It comes in a rugged aluminum enclosure with wall mounting support. The data is exported to the uRADMonitor network with Wifi or LORAWAN, and can be accessed in real time using the cloud API interface or directly via the local network.
Andy, UK: "I saw your project on the EEVBlog, absolutely fantastic bit of kit."
| Sensor | Parameter | Minimum value | Maximum value |
| MEMs | Temperature | -40 °C | +85 °C |
| Humidity | 0% RH | 100% RH | |
| Laser scattering | PM1.0 | 0 μg/m³ | 1000 μg/m³ |
| PM2.5 | 0 μg/m³ | 1000 μg/m³ | |
| PM10 | 0 μg/m³ | 1000 μg/m³ | |
| MOX | VOC | 10 ppm | 1000 ppm * |
| Analogue sound sensor | Noise level | 30dB | 130dB |
| Electrochemical | Ozone | 0 ppm | 10 ppm |
| Electrochemical | Nitrogen Dioxide | 0 ppm | 10 ppm |
| Electrochemical | Sulphur Dioxide | 0 ppm | 20 ppm |
| Electrochemical | Carbon Monoxide | 0 ppm | 200 ppm |
Custom gases detection options
* The unit comes with Ozone, Nitrogen Dioxide, Sulphur Dioxide and Carbon Monoxide calibrated electrochemical sensors built in. These 4 sensors, can be replaced with any combination of sensors for the below gases and ranges. In some situations we can offer custom detection intervals (eg. 0-1000ppm for CO, etc):| Symbol | Gas | Detection interval |
| CO | Carbon monoxide | 0 - 200ppm |
| O2 | Oxigen | 0 - 25%VOL |
| NH3 | Ammonia gas | 0 - 100ppm |
| H2S | Hydrogen sulfide | 0 - 100ppm |
| SO2 | Sulfur dioxide | 0 - 20ppm |
| NO2 | Nitrogen dioxide | 0 - 10ppm |
| Cl2 | Chlorine gas | 0 - 20ppm |
| O3 | Ozone | 0 - 10ppm |
| H2 | Hydrogen gas | 0 - 1000ppm |
| HF | Hydrogen fluoride | 0 - 10ppm |
| C2H4 | Ethylene | 0 - 100ppm |
| CH2O | Formaldehyde | 0 - 10ppm |
| ETO | Ethylene oxide | 0 - 20ppm |
| HCl | Hydrogen chloride | 0 - 20ppm |
| C6H6 | Benzene | 0 - 100ppm |
| C7H8 | Toluene | 0 - 500ppm |
| C2H3Cl | Vinyl chloride | 0 - 20ppm |
| C2H6S | Methyl Sulfide | 0 - 100ppm |
| C2H6S2 | Dimethyl Disulfide | 0 - 100ppm |
| AsH3 | Arsine | 0 - 3ppm |
| C3H9N | Trimethylamine | 0 - 100ppm |
| C8H8 | Styrene | 0 - 100ppm |
| CH4S | Methanethiol | 0 - 100ppm |
| CS2 | Carbon Disulfide | 0 - 100ppm |
| PH3 | Phosphine | 0 - 10ppm |
| HCN | Hydrogen cyanide | 0 - 100ppm |
The National Emissions Ceiling Directive caps some emissions including particulate matter (PM) and nitrogen dioxide (NOx) at national level. A revised version of the directive is as of 2016 under scrutiny by the Council of Ministers and European Parliament. Across the EU in 2013, nitrogen dioxide (NO2), which is mostly produced by traffic, caused 68,000 premature deaths. The Dieselgate scandal exposed how Volkswagen had gamed NO2 emissions tests.
Ozone (O3) killed 16,000 and small particulate matter (PM2.5) caused 436,000 deaths in the same year. PM2.5 particles, microscopic specks of dust and soot caused by burning fossil fuels, can enter the lungs and bloodstream.
 Air pollution has different particulate matter (PM) components – smoke, dirt and dust form coarse particles known as PM10 and metals and toxic exhaust from smelting, vehicle exhaust, power plants and refuse burning forming fine particles called PM2.5.
Air pollution has different particulate matter (PM) components – smoke, dirt and dust form coarse particles known as PM10 and metals and toxic exhaust from smelting, vehicle exhaust, power plants and refuse burning forming fine particles called PM2.5.uRADMonitor INDUSTRIAL is intended for the industrial sector where medium and high gas concentrations need to be observed.
| Item | Parameter | Ratings |
| Voltage | External | 6V - 24V |
| Connectivity | 4 options | WiFi, LoRaWAN, USB |
| Microcontroller | Atmega1284p | 8 bit |
If you have a radio variant with an antenna, connect the antenna first. Next, connect the uRADMonitor to the power source using a DC adapter with voltage between 6V and 24V. Connect the uRADMonitor to the power source using a DC adapter with voltage between 6V and 24V or via the USB port with a 5V USB adapter.
The WIFI variant
Use a smartphone or a computer with WLAN capabilities to connect to the local hotspot spawned by your unit. The SSID is uRADMonitor-XX, where XX are the last two digits of the Device ID number. The key is the Device ID, in uppercase, as printed on the enclosure. Open 192.168.4.1 in your browser, and click the "WIFI" link to setup the connection to the Internet AP. Enter the SSID and key of your Internet Access Point. If the connection fails, you will see the status message, and three consecutive beeps will indicated the problem. Alternatively you can change the configuration using the USB data port. See the USB manual.
The LORAWAN variant
Your device must be pre-provisioned with the LoraWAN Gateway and network server details. Alternativelly you can change the configuration using the USB data port. See the USB manual.
The device comes with an USB port, so the configuration settings can be customized via the USB connection. Consult your product manual for complete details.
Technical Datasheet
USB Serial Commands Manual
LoRaWAN Data Server Callback manual
Product Limited Warranty terms
uRADMonitor with WIFI Configuration manual
Data access:
Terms of Service
API Terms of Service
API and Server Specs
Direct Data access
Payload Structure (FW78)
USB Driver
In their recent versions, Windows, Linux and MacOS come with built in drivers for your uRADMonitor unit. Use the following resources only if the built in drivers do not work for you.
USB Driver for Windows
USB Driver for Linux
USB Driver for MacOS
RO:
Fisa tehnica
SMOGGIE-PMContact us
This is an ultra-low cost automated air quality monitor with a Stevenson rain proof enclosure and a simple mount system to make installation easy. It features a high quality laser scatering Particulate Matter sensor for PM1, PM2.5 and PM10 and an additional sensor for temperature and humidity. It connects to the internet via Wifi and can be powered by a standard 5V micro-usb cable. Readings are accessed via the uRADMonitor API or decentralized via your local network. This monitor is lab tested for data accuracy.
Ron, Canada: "I really appreciate all the hard work you have put into this project over the years and wish you continued success."
| Sensor | Parameter | Minimum value | Maximum value |
| MEMs | Temperature | -40 °C | +85 °C |
| Humidity | 0% RH | 100% RH | |
| Laser scattering | PM1.0 | 0 μg/m³ | 1000 μg/m³ |
| PM2.5 | 0 μg/m³ | 1000 μg/m³ | |
| PM10 | 0 μg/m³ | 1000 μg/m³ |

[1] Volatile Organic Compounds’ Impact on Indoor Air Quality, US Environmental Protection Agency

| Item | Parameter | Ratings |
| Voltage | External | 5V micro-USB |
| Consumption | Current | 80mA (max 120mA with fan running) |
| Connectivity | Internet | Wifi |
| Microcontroller | ESP8266 | 8 bit |
| Enclosure | Rainproof plastic | 42x43x27 mm |
Quick Setup
Connect it to power using a 5V micro-USB cable. Use a smartphone or a computer with WLAN capabilities to connect to the local hotspot spawned by your SMOGGIE unit. The SSID is uRADMonitor-XX, where XX are the last two digits of the Device ID number. The key is the Device ID, in uppercase, as printed on the enclosure. Open 192.168.4.1 in your browser, and click the "WIFI/CONFIG" link to setup the connection to the Internet AP. Enter the SSID and key of your Internet Access Point. If the connection fails, you will see the status message.
USB configuration
Alternatively, the SSID and KEY can also be configured via USB. Connect to your SMOGGIE via USB, baudrate 9600bps, open a terminal program and type the two commands: "key1","SSID" then "key2","your WLAN key" . Use the "getsettings" command to verify that the new settings are in place. Quotes are a must, and there are no spaces in between. See the USB Commands manual for more. See the USB Commands manual if you need more help.
Technical Datasheet
USB Serial Commands Manual
Product Limited Warranty terms
uRADMonitor with WIFI Configuration manual
Open source on Github
Data access:
Terms of Service
API Terms of Service
API and Server Specs
Direct Data access
Accuracy lab tests and certifications
AQMD AQ-SPEC Performance test report
USB Driver
In their recent versions, Windows, Linux and MacOS come with built in drivers for your uRADMonitor unit. Use the following resources only if the built in drivers do not work for you.
USB Driver for Windows
USB Driver for Linux
USB Driver for MacOS
RO:
Fisa tehnica
RO: Ghid rapid de utilizare
RO: conditii generale de utilizare
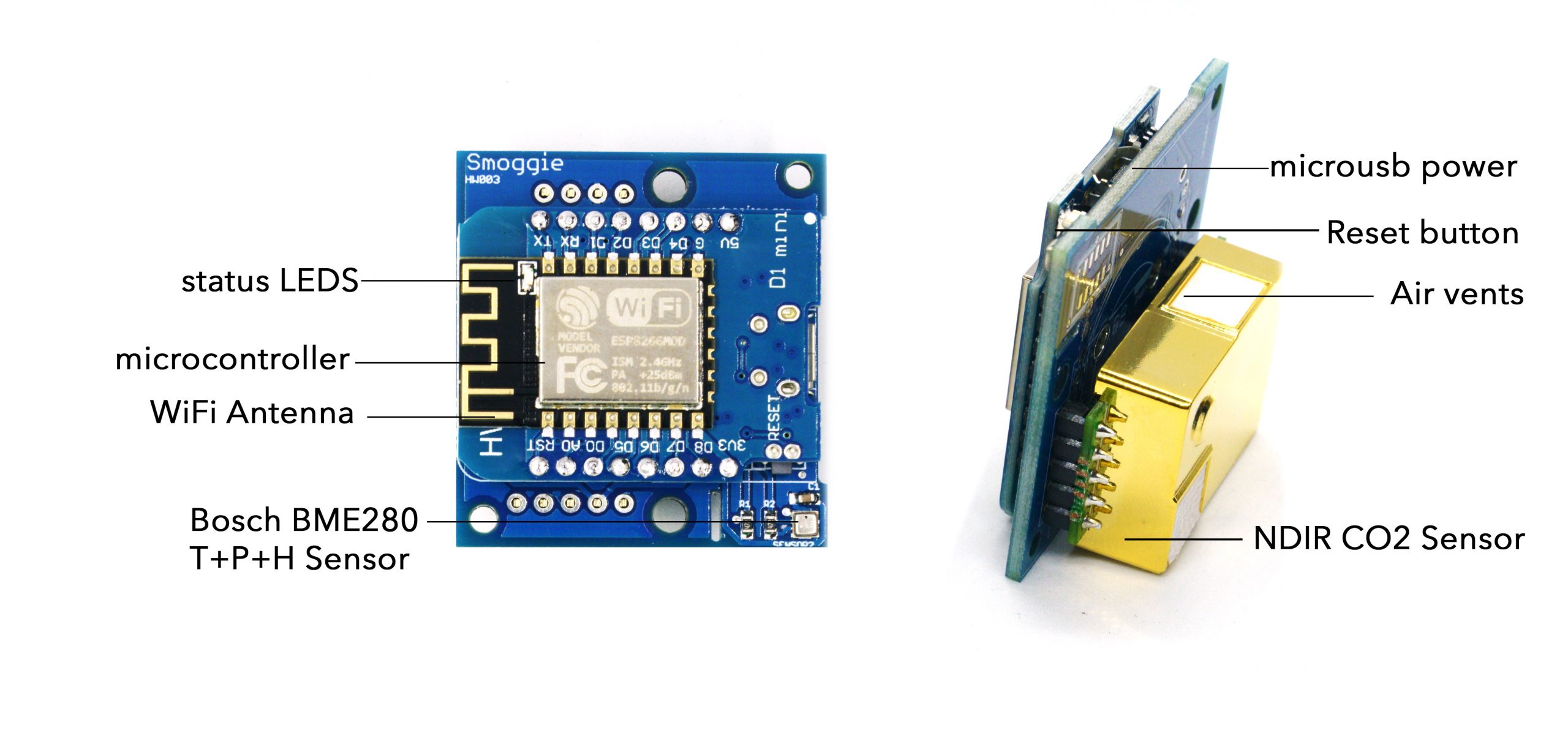
SMOGGIE-CO2Contact us
This is a compact, automated Carbon Dioxide monitor. It detects CO2 levels in your home via a high quality non dispersive infrared sensor. Carbon dioxide at levels that are unusually high indoors may cause occupants to grow drowsy, to get headaches, or to function at lower activity levels. There is an additional sensor for temperature and humidity. It connects to the internet via Wifi and can be powered by a standard 5V micro-usb cable. It provides real time 24/7 CO2 monitoring that can help you improve your indoor air quality.
Steve, Canada: "Your design is beautiful and the concept is brilliant. It's somewhat surprising that it hasn't been done before (by civilians). All survey meters should be connected to the net. Maybe in the next generation of smart appliances."
| Sensor | Parameter | Minimum value | Maximum value |
| MEMs | Temperature | -40 °C | +85 °C |
| Humidity | 0% RH | 100% RH | |
| NDIR CO2 Sensor | CO2 | 400 ppm | 5000 ppm |
Carbon Dioxide is a contributing factor to the Sick building syndrome (SBS), a medical condition where people in a building suffer from symptoms of illness or feel unwell for no apparent reason. The symptoms tend to increase in severity with the time people spend in the building, and improve over time or even disappear when people are away from the building. The main identifying observation is an increased incidence of complaints of symptoms such as headache, eye, nose, and throat irritation, fatigue, and dizziness and nausea. These symptoms appear to be linked to time spent in a building, though no specific illness or cause can be identified. A 1984 World Health Organization (WHO) report suggested up to 30% of new and remodeled buildings worldwide may be subject of complaints related to poor indoor air quality.
In homes and offices:
A 100 ppm increase in indoor CO2 concentration was significantly associated with headache (..). Office workers exposed to indoor CO2 concentrations higher than 800 ppm reported a significant increase in eye irritation and upper respiratory symptoms . A 100 ppm increase in dCO2 in the range from 467 to 2800 ppm in indoor CO2 was significantly associated with dry throat, tiredness, and dizziness (417 participants from 87 offices) (Lu et al., 2015). A 100 ppm increase in CO2 concentration (range, 549–1318 ppm) was positively correlated with non-specific symptoms including headache and dizziness (107 participants from 11 offices) although the correlation was not significant (Azuma et al., 2018).
Twenty-two participants were exposed to CO2 at 600, 1000, and 2500 ppm (three 2.5-h sessions, one day; artificially elevated CO2 concentrations) in an office-like chamber. Statistically significant decrements occurred in cognitive performance (decision making, problem resolution) starting at 1000 ppm (Satish et al., 2012).

In schools:
A study in schoolchildren exposed to indoor CO2 concentrations higher than 1000 ppm showed significantly higher risk for dry cough and rhinitis (654 children of 46 classrooms) but outdoor air flow rate per person was inversely correlated with indoor CO2 concentrations (Simoni et al., 2010). A 200 ppm increase in indoor CO2 concentration (range, 1000–2000 ppm) in 45 day care centers (DCCs) was significantly associated with reported wheezing in the 3186 attending children, and a positive trend was observed between CO2 concentration and the prevalence of asthma.
[1] Effects of low-level inhalation exposure to carbon dioxide in indoor environments
The units can be managed via the dashboard. The data can be viewed remotely on a computer or on a mobile device.
| Item | Parameter | Ratings |
| Voltage | External | 5V micro-USB |
| Consumption | Current | 80mA (max 170mA on CO2 pulse) |
| Connectivity | Internet | Wifi |
| Microcontroller | ESP8266 | 8 bit |
| Enclosure | Rainproof plastic | 42x43x27 mm |
Quick Setup
Connect it to power using a 5V micro-USB cable. Use a smartphone or a computer with WLAN capabilities to connect to the local hotspot spawned by your SMOGGIE unit. The SSID is uRADMonitor-XX, where XX are the last two digits of the Device ID number. The first time, the key is the Device ID, in uppercase, as printed on the enclosure. You can change this key later. Open 192.168.4.1 in your browser, and click the "WIFI/CONFIG" link to setup the connection to the Internet AP. Select the SSID and enter the key of your Internet Access Point.
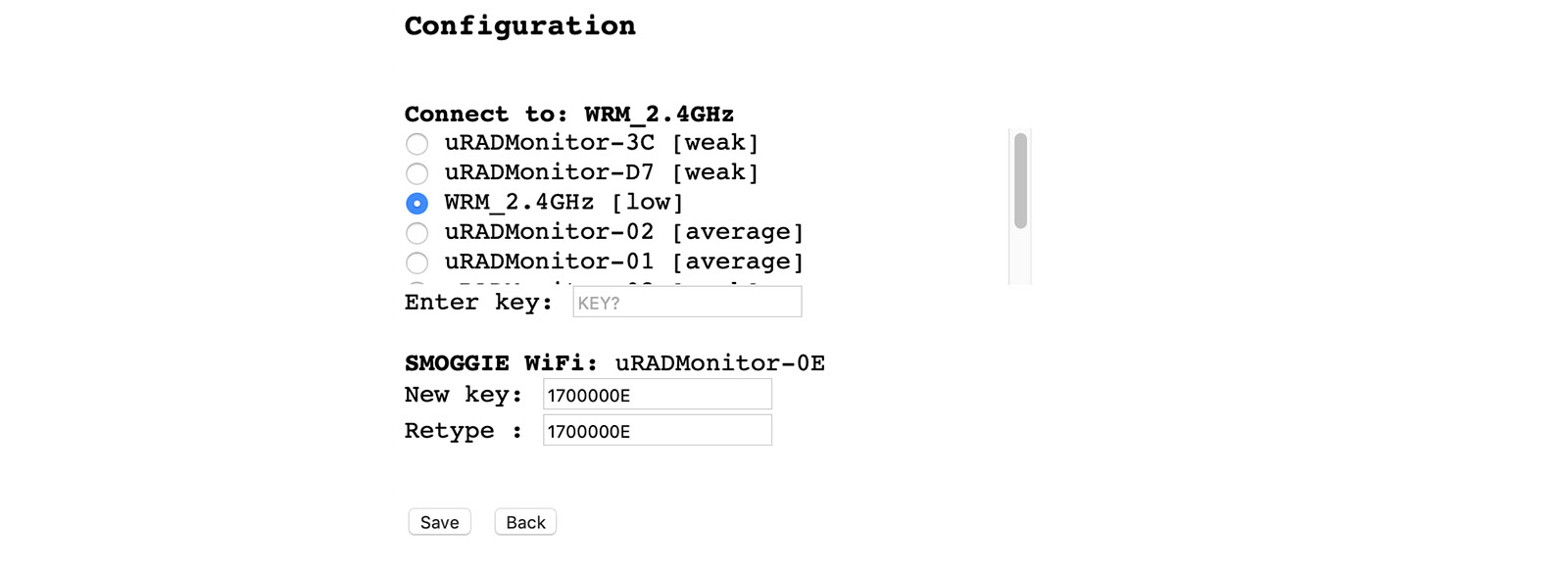
USB configuration
Alternatively, the SSID and KEY can also be configured via USB. Connect to your SMOGGIE via USB, baudrate 9600bps, open a terminal program and type the two commands: "key1","SSID" then "key2","your WLAN key" . Use the "getsettings" command to verify that the new settings are in place. Quotes are a must, and there are no spaces in between. See the USB Commands manual for more. See the USB Commands manual if you need more help.
Technical Datasheet
USB Serial Commands Manual
Product Limited Warranty terms
uRADMonitor with WIFI Configuration manual
Open source on Github
Data access:
Terms of Service
API Terms of Service
API and Server Specs
Direct Data access
USB Driver
In their recent versions, Windows, Linux and MacOS come with built in drivers for your uRADMonitor unit. Use the following resources only if the built in drivers do not work for you.
USB Driver for Windows
USB Driver for Linux
USB Driver for MacOS
RO:
Fisa tehnica
RO: Ghid rapid de utilizare
RO: conditii generale de utilizare
SMOGGIE-GASContact us
This is a compact, automated GAS monitor. It accommodates a single electrochemical cell that you can choose to match the gas you need to detect. Currently we support H2S, CO, O2, NH3, NO2, HF, SO2, CL2, O3, H2, HCL. There is an additional sensor for temperature and humidity. It connects to the internet via Wifi and can be powered by a standard 5V micro-usb cable. It provides real time 24/7 monitoring for toxic gases.
Manuel, Germany: "Please keep that project going, it is a great idea and all people may benefit from it :)"
| Sensor | Parameter | Resolution | Minimum value | Maximum value |
| MEMs | Temperature | 0.5°C | -40 °C | +85 °C |
| Humidity | 1%RH | 0%RH | 100% RH | |
| Electrochemical Sensor | H2S | 0.1 ppm | 0 ppm | 100 ppm |
| O3 | 0.1 ppm | 0 ppm | 10 ppm | |
| NO2 | 0.1 ppm | 0 ppm | 10 ppm | |
| SO2 | 0.1 ppm | 0 ppm | 20 ppm | |
| CO | 1 ppm | 0 ppm | 200 ppm |
By the nature of the technology used, any sensor can potentially fail to meet specification without warning. We make every effort to ensure reliability of all sensors but where life safety is a performance requirement of the product and, where practical, we recommend that all gas sensors and instruments using sensors are checked for response to gas before use. We accept no liability for any consequential losses, injury or damage resulting from the use of the uRADMonitor products. Customers should test the sensors under their own conditions to ensure that the sensors are suitable for their own requirements and in accordance with the plans and circumstances of the specified project and any standards / regulations pertaining to the country in which the sensors will be utilized.
Hydrogen sulfide is often produced from the microbial breakdown of organic matter in the absence of oxygen gas, such as in swamps and sewers; this process is commonly known as anaerobic digestion which is done by sulfate-reducing microorganisms. H2S also occurs in volcanic gases, natural gas, and in some sources of well water. [1]
Hydrogen sulfide is a broad-spectrum poison, meaning that it can poison several different systems in the body, although the nervous system is most affected. The toxicity of H2S is comparable with that of carbon monoxide. It binds with iron in the mitochondrial cytochrome enzymes, thus preventing cellular respiration. Since hydrogen sulfide occurs naturally in the body, the environment, and the gut, enzymes exist to detoxify it. At some threshold level, believed to average around 300–350 ppm, the oxidative enzymes become overwhelmed. Many personal safety gas detectors, such as those used by utility, sewage and petrochemical workers, are set to alarm at as low as 5 to 10 ppm and to go into high alarm at 15 ppm. Detoxification is effected by oxidation to sulfate, which is harmless. Hence, low levels of hydrogen sulfide may be tolerated indefinitely.
Exposure to lower concentrations can result in eye irritation, a sore throat and cough, nausea, shortness of breath, and fluid in the lungs (pulmonary edema). These effects are believed to be due to the fact that hydrogen sulfide combines with alkali present in moist surface tissues to form sodium sulfide, a caustic. These symptoms usually go away in a few weeks.
Long-term, low-level exposure may result in fatigue, loss of appetite, headaches, irritability, poor memory, and dizziness. Chronic exposure to low level H2S (around 2 ppm) has been implicated in increased miscarriage and reproductive health issues among Russian and Finnish wood pulp workers, but the reports have not (as of circa 1995) been replicated.
Short-term, high-level exposure can induce immediate collapse, with loss of breathing and a high probability of death. If death does not occur, high exposure to hydrogen sulfide can lead to cortical pseudolaminar necrosis, degeneration of the basal ganglia and cerebral edema. Although respiratory paralysis may be immediate, it can also be delayed up to 72 hours.
Carbon monoxide (CO) is a colorless, odorless, and tasteless flammable gas that is slightly less dense than air. It is toxic to animals that use hemoglobin as an oxygen carrier (both Invertebrate and vertebrate) when encountered in concentrations above about 35 ppm, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal biological functions. In the atmosphere, it is spatially variable and short lived, having a role in the formation of ground-level ozone.[2] Carbon monoxide poisoning is the most common type of fatal air poisoning in many countries. Carbon monoxide is colorless, odorless, and tasteless, but highly toxic. It combines with hemoglobin to produce carboxyhemoglobin, which usurps the space in hemoglobin that normally carries oxygen, but is ineffective for delivering oxygen to bodily tissues. Concentrations as low as 667 ppm may cause up to 50% of the body's hemoglobin to convert to carboxyhemoglobin. A level of 50% carboxyhemoglobin may result in seizure, coma, and fatality. In the United States, the OSHA limits long-term workplace exposure levels above 50 ppm.
The most common symptoms of carbon monoxide poisoning may resemble other types of poisonings and infections, including symptoms such as headache, nausea, vomiting, dizziness, fatigue, and a feeling of weakness. Affected families often believe they are victims of food poisoning. Infants may be irritable and feed poorly. Neurological signs include confusion, disorientation, visual disturbance, syncope (fainting), and seizures.
Some descriptions of carbon monoxide poisoning include retinal hemorrhages, and an abnormal cherry-red blood hue. In most clinical diagnoses these signs are seldom noticed. One difficulty with the usefulness of this cherry-red effect is that it corrects, or masks, what would otherwise be an unhealthy appearance, since the chief effect of removing deoxygenated hemoglobin is to make an asphyxiated person appear more normal, or a dead person appear more lifelike, similar to the effect of red colorants in embalming fluid. The "false" or unphysiologic red-coloring effect in anoxic CO-poisoned tissue is related to the meat-coloring commercial use of carbon monoxide, discussed below.
Carbon monoxide also binds to other molecules such as myoglobin and mitochondrial cytochrome oxidase. Exposures to carbon monoxide may cause significant damage to the heart and central nervous system, especially to the globus pallidus, often with long-term chronic pathological conditions. Carbon monoxide may have severe adverse effects on the fetus of a pregnant woman.
[1] Hydrogen sulfide
[2] Carbon monoxide
The units can be managed via the dashboard. The data can be viewed remotely on a computer or on a mobile device.
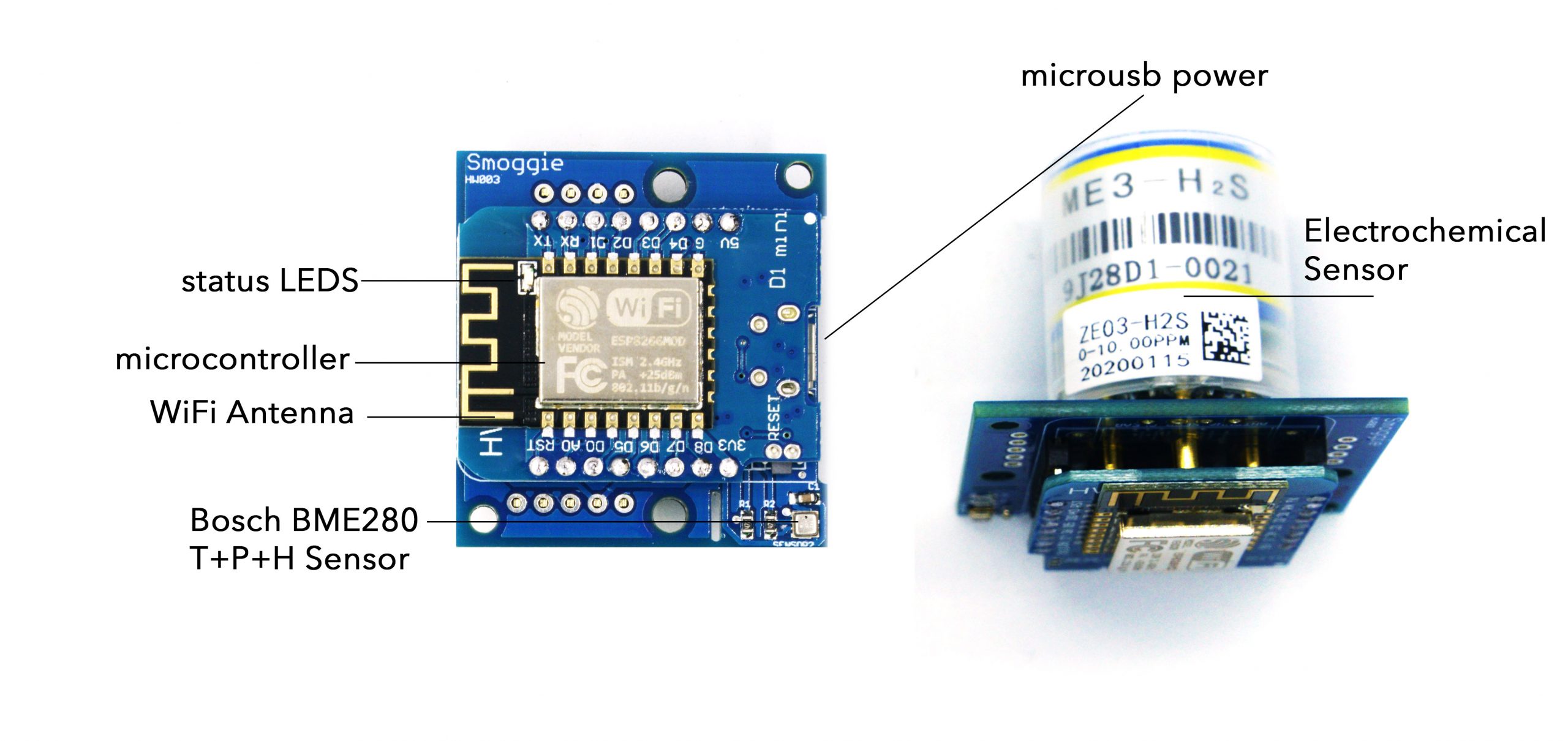
| Item | Parameter | Ratings |
| Voltage | External | 5V micro-USB |
| Consumption | Current | 80mA |
| Connectivity | Internet | Wifi |
| Microcontroller | ESP8266 | 8 bit |
| Enclosure | Rainproof plastic | 42x43x47 mm |
Quick Setup
Connect it to power using a 5V micro-USB cable. Use a smartphone or a computer with WLAN capabilities to connect to the local hotspot spawned by your SMOGGIE unit. The SSID is uRADMonitor-XX, where XX are the last two digits of the Device ID number. The key is the Device ID, in uppercase, as printed on the enclosure. You can change this key later. Open 192.168.4.1 in your browser, and click the "WIFI/CONFIG" link to setup the connection to the Internet AP. Select the SSID and enter the key of your Internet Access Point.

USB configuration
Alternatively, the SSID and KEY can also be configured via USB. Connect to your SMOGGIE via USB, baudrate 9600bps, open a terminal program and type the two commands: "key1","SSID" then "key2","your WLAN key" . Use the "getsettings" command to verify that the new settings are in place. Quotes are a must, and there are no spaces in between. See the USB Commands manual for more. See the USB Commands manual if you need more help.
Technical Datasheet
USB Serial Commands Manual
Product Limited Warranty terms
uRADMonitor with WIFI Configuration manual
Open source on Github
Data access:
Terms of Service
API Terms of Service
API and Server Specs
Direct Data access
USB Driver
In their recent versions, Windows, Linux and MacOS come with built in drivers for your uRADMonitor unit. Use the following resources only if the built in drivers do not work for you.
USB Driver for Windows
USB Driver for Linux
USB Driver for MacOS
RO:
RO: Ghid rapid de utilizare
RO: conditii generale de utilizare
MODEL CITYContact us
This automated, fixed monitoring station for urban environmental monitoring offers unmatched 1PPB resolution for gases, so it is capable of detecting even the slightest changes in ambient air composition. It tracks a total of 10 important air quality parameters including Particulate Matter, Carbon Monoxide, Ozone, Sulphur Dioxide, Nitrogen Dioxide. It comes in a rain proof Stevenson enclosure ready for outdoor use. The data can be accessed in real time using the cloud API interface or directly via the local network.
Swaleh, Mauritius: "You have a good vision for the wellness of mankind, your effort will pay and mankind will benefit."
| Sensor | Parameter | Minimum value | Maximum value |
| MEMS | Temperature | -40 °C | +85 °C |
| Humidity | 0% RH | 100% RH | |
| Barometric Pressure | 300 hPa | 1100 hPa | |
| Laser Scattering | PM1.0 | 0 μg/m³ | 1000 μg/m³ |
| PM2.5 | 0 μg/m³ | 1000 μg/m³ | |
| PM10 | 0 μg/m³ | 1000 μg/m³ | |
| Electrochemical 1ppb | Ozone | 0 ppm | 1ppm |
| Electrochemical 1ppb | Nitrogen Dioxide | 0 ppm | 1 ppm |
| Electrochemical 1ppb | Sulphur Dioxide | 0 ppm | 1 ppm |
| Electrochemical 1ppb | Carbon Monoxide | 0 ppm | 10 ppm |
The National Emissions Ceiling Directive caps some emissions including particulate matter (PM) and nitrogen dioxide (NOx) at national level. A revised version of the directive is as of 2016 under scrutiny by the Council of Ministers and European Parliament. Across the EU in 2013, nitrogen dioxide (NO2), which is mostly produced by traffic, caused 68,000 premature deaths. The Dieselgate scandal exposed how Volkswagen had gamed NO2 emissions tests.
Ozone (O3) killed 16,000 and small particulate matter (PM2.5) caused 436,000 deaths in the same year. PM2.5 particles, microscopic specks of dust and soot caused by burning fossil fuels, can enter the lungs and bloodstream.
 Air pollution has different particulate matter (PM) components – smoke, dirt and dust form coarse particles known as PM10 and metals and toxic exhaust from smelting, vehicle exhaust, power plants and refuse burning forming fine particles called PM2.5.
Air pollution has different particulate matter (PM) components – smoke, dirt and dust form coarse particles known as PM10 and metals and toxic exhaust from smelting, vehicle exhaust, power plants and refuse burning forming fine particles called PM2.5.uRADMonitor CITY is equipped with all sensors required to compute the Air Quality Index as defined by several international standards on air quality and give a direct assessment on the pollution problem and possible infringements.

Picture: uRADMonitor CITY installed outdoors
If you have a radio variant with an antenna, connect the antenna first. Next, connect the uRADMonitor to the power source. Be careful when working with mains voltage. Alternativelly you can connect the uRADMonitor to the power source using a DC adapter with voltage between 6V and 24V or via the USB port with a 5V USB adapter. The device comes with an USB port, so the configuration settings can be customized via the USB connection. Consult your product manual for how to use the USB connection.
Use a smartphone or a computer with WLAN capabilities to connect to the local hotspot spawned by your unit. The SSID is uRADMonitor-XX, where XX are the last two digits of the Device ID number. The key is the Device ID, in uppercase, as printed on the enclosure. Open 192.168.4.1 in your browser, and click the "WIFI" link to setup the connection to the Internet AP. Enter the SSID and key of your Internet Access Point. Read more in the WIFI configuration manual.
Configure the LoRaWAN network server details via USB. See the USB manual.
This goes plug and play as it only needs an Internet Router with DHCP enabled : the uRADMonitor CITY will receive an IP automatically and stard broadcasting data without any other configuration requirements.
Technical Datasheet
USB Serial Commands Manual
LoRaWAN Data Server Callback manual
API and Server Specs
Product Limited Warranty terms
uRADMonitor with WIFI Configuration manual
Accuracy lab tests and certifications
AIRPARIF France AIRLAB 2023 Lab tests
USB Driver
In their recent versions, Windows, Linux and MacOS come with built in drivers for your uRADMonitor unit. Use the following resources only if the built in drivers do not work for you.
USB Driver for Windows
USB Driver for Linux
USB Driver for MacOS
RO:
Fisa tehnica
MODEL seeRADONContact us
seeRADON continues uRADMonitor’s now established tradition to venture into the invisible, delivering real time data on the chemical and physical surrounding factors that can harm your health. This time, seeRADON is a modern IOT sensor that tracks the radioactive gas RADON. It features a solid state sensor for detection of Radon daughters’ alpha particles. Combined with the WIFI connectivity you’ll be able to monitor your RADON levels in real time.
Martin, UK: "Congratulations on an excellent idea and brilliant implementation!"
| Sensor | Parameter | Minimum value | Maximum value |
| MEMS | Temperature | -40 °C | +125 °C |
| Humidity | 0% RH | 100% RH | |
| Semiconductor | Radon | 0.1 pCi/L | 1750 pCi/L |
| Item | Parameter | Ratings |
| Voltage | External | 5V micro-USB |
| Consumption | Current | 50mA |
| Connectivity | Internet | Wifi |
| Microcontroller | ESP8266 | 8 bit |
| Enclosure | Rainproof plastic | 85x85x50 mm 100grams |
Quick Setup
Connect it to power using a 5V micro-USB cable. Use a smartphone or a computer with WLAN capabilities to connect to the local hotspot spawned by your seeRADON unit. The SSID is uRADMonitor-XX, where XX are the last two digits of the Device ID number. The key is the Device ID, in uppercase, as printed on the enclosure. Open 192.168.4.1 in your browser, and click the "WIFI/CONFIG" link to setup the connection to the Internet AP. Enter the SSID and key of your Internet Access Point. If the connection fails, you will see the status message.
USB configuration
Alternatively, the SSID and KEY can also be configured via USB. Connect to your seeRADON via USB, baudrate 9600bps, open a terminal program and type the two commands: "key1","SSID" then "key2","your WLAN key" . Use the "getsettings" command to verify that the new settings are in place. Quotes are a must, and there are no spaces in between. See the USB Commands manual for more. See the USB Commands manual if you need more help.
Technical Datasheet
USB Serial Commands Manual
API and Server Specs
Product Limited Warranty terms
Decentralized unit use for direct data access
uRADMonitor with WIFI Configuration manual
RO: Ghid rapid de utilizare
RO: conditii generale de utilizare
USB Driver
In their recent versions, Windows, Linux and MacOS come with built in drivers for your uRADMonitor unit. Use the following resources only if the built in drivers do not work for you.
USB Driver for Windows
USB Driver for Linux
USB Driver for MacOS
MODEL BUZZContact us
Industrial grade Noise meter with a WIFI connection to report sound level measurements in real time. The data can be accessed via the API interface or directly via the local network.
Elia, Germany: "hope that your project will be successful and that the network will continue to grow!"
| Sensor | Parameter | Minimum value | Maximum value |
| MEMS | Temperature | -40 °C | +125 °C |
| Humidity | 0% RH | 100% RH | |
| SM7901 | Noise | 30 dB | 130 dB |

| Item | Parameter | Ratings |
| Voltage | External | 5V micro-USB |
| Consumption | Current | 50mA |
| Connectivity | Internet | Wifi |
| Microcontroller | ESP8266 | 8 bit |
| Enclosure | Rainproof plastic | 85x44x20 mm |
Quick Setup
Connect it to power using a 5V micro-USB cable. Use a smartphone or a computer with WLAN capabilities to connect to the local hotspot spawned by your BUZZ unit. The SSID is uRADMonitor-XX, where XX are the last two digits of the Device ID number. The key is the Device ID, in uppercase, as printed on the enclosure. Open 192.168.4.1 in your browser, and click the "WIFI/CONFIG" link to setup the connection to the Internet AP. Enter the SSID and key of your Internet Access Point. If the connection fails, you will see the status message.
USB configuration
Alternatively, the SSID and KEY can also be configured via USB. Connect to your BUZZ via USB, baudrate 9600bps, open a terminal program and type the two commands: "key1","SSID" then "key2","your WLAN key" . Use the "getsettings" command to verify that the new settings are in place. Quotes are a must, and there are no spaces in between. See the USB Commands manual for more. See the USB Commands manual if you need more help.
Technical Datasheet
USB Serial Commands Manual
API and Server Specs
Product Limited Warranty terms
Decentralized unit use for direct data access
uRADMonitor with WIFI Configuration manual
RO: Ghid rapid de utilizare
RO: conditii generale de utilizare
USB Driver
In their recent versions, Windows, Linux and MacOS come with built in drivers for your uRADMonitor unit. Use the following resources only if the built in drivers do not work for you.
USB Driver for Windows
USB Driver for Linux
USB Driver for MacOS
SENSIGASContact us
The sensitivity of this sensor is unmatched: With this automated sensor you can monitor the gas you choose down to 1PPB resolution, so this will pick up even the gas traces in ambient air composition. It accommodates a single electrochemical cell that you can choose to match the gas you need to detect. Currently we support H2S, CO, NO2, SO2 and O3. There is an additional embedded sensor for temperature and humidity. It connects to the internet via WIFI and can be powered by a standard 5V micro-usb cable. It provides real time 24/7 monitoring for toxic gases.
Andrei A., Romania: The most accurate and widespread air quality monitoring network. I also own several of their sensors and all work flawlessly.
| Sensor | Parameter | Resolution | Minimum value | Maximum value |
| MEMs | Temperature | 0.5°C | -40 °C | +85 °C |
| Humidity | 1%RH | 0%RH | 100% RH | |
| Electrochemical Sensor | H2S | 1 ppb | 1 ppb | 1 ppm |
| O3 | 1 ppb | 1 ppb | 1 ppm | |
| NO2 | 1 ppb | 1 ppb | 1 ppm | |
| SO2 | 1 ppb | 1 ppb | 1 ppm | |
| CO | 1 ppb | 1 ppb | 10 ppm |
By the nature of the technology used, any sensor can potentially fail to meet specification without warning. We make every effort to ensure reliability of all sensors but where life safety is a performance requirement of the product and, where practical, we recommend that all gas sensors and instruments using sensors are checked for response to gas before use. We accept no liability for any consequential losses, injury or damage resulting from the use of the uRADMonitor products. Customers should test the sensors under their own conditions to ensure that the sensors are suitable for their own requirements and in accordance with the plans and circumstances of the specified project and any standards / regulations pertaining to the country in which the sensors will be utilized.
Hydrogen sulfide is often produced from the microbial breakdown of organic matter in the absence of oxygen gas, such as in swamps and sewers; this process is commonly known as anaerobic digestion which is done by sulfate-reducing microorganisms. H2S also occurs in volcanic gases, natural gas, and in some sources of well water. [1]
Hydrogen sulfide is a broad-spectrum poison, meaning that it can poison several different systems in the body, although the nervous system is most affected. The toxicity of H2S is comparable with that of carbon monoxide. It binds with iron in the mitochondrial cytochrome enzymes, thus preventing cellular respiration. Since hydrogen sulfide occurs naturally in the body, the environment, and the gut, enzymes exist to detoxify it. At some threshold level, believed to average around 300–350 ppm, the oxidative enzymes become overwhelmed. Many personal safety gas detectors, such as those used by utility, sewage and petrochemical workers, are set to alarm at as low as 5 to 10 ppm and to go into high alarm at 15 ppm. Detoxification is effected by oxidation to sulfate, which is harmless. Hence, low levels of hydrogen sulfide may be tolerated indefinitely.
Exposure to lower concentrations can result in eye irritation, a sore throat and cough, nausea, shortness of breath, and fluid in the lungs (pulmonary edema). These effects are believed to be due to the fact that hydrogen sulfide combines with alkali present in moist surface tissues to form sodium sulfide, a caustic. These symptoms usually go away in a few weeks.
Long-term, low-level exposure may result in fatigue, loss of appetite, headaches, irritability, poor memory, and dizziness. Chronic exposure to low level H2S (around 2 ppm) has been implicated in increased miscarriage and reproductive health issues among Russian and Finnish wood pulp workers, but the reports have not (as of circa 1995) been replicated.
Short-term, high-level exposure can induce immediate collapse, with loss of breathing and a high probability of death. If death does not occur, high exposure to hydrogen sulfide can lead to cortical pseudolaminar necrosis, degeneration of the basal ganglia and cerebral edema. Although respiratory paralysis may be immediate, it can also be delayed up to 72 hours.
Carbon monoxide (CO) is a colorless, odorless, and tasteless flammable gas that is slightly less dense than air. It is toxic to animals that use hemoglobin as an oxygen carrier (both Invertebrate and vertebrate) when encountered in concentrations above about 35 ppm, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal biological functions. In the atmosphere, it is spatially variable and short lived, having a role in the formation of ground-level ozone.[2] Carbon monoxide poisoning is the most common type of fatal air poisoning in many countries. Carbon monoxide is colorless, odorless, and tasteless, but highly toxic. It combines with hemoglobin to produce carboxyhemoglobin, which usurps the space in hemoglobin that normally carries oxygen, but is ineffective for delivering oxygen to bodily tissues. Concentrations as low as 667 ppm may cause up to 50% of the body's hemoglobin to convert to carboxyhemoglobin. A level of 50% carboxyhemoglobin may result in seizure, coma, and fatality. In the United States, the OSHA limits long-term workplace exposure levels above 50 ppm.
The most common symptoms of carbon monoxide poisoning may resemble other types of poisonings and infections, including symptoms such as headache, nausea, vomiting, dizziness, fatigue, and a feeling of weakness. Affected families often believe they are victims of food poisoning. Infants may be irritable and feed poorly. Neurological signs include confusion, disorientation, visual disturbance, syncope (fainting), and seizures.
Some descriptions of carbon monoxide poisoning include retinal hemorrhages, and an abnormal cherry-red blood hue. In most clinical diagnoses these signs are seldom noticed. One difficulty with the usefulness of this cherry-red effect is that it corrects, or masks, what would otherwise be an unhealthy appearance, since the chief effect of removing deoxygenated hemoglobin is to make an asphyxiated person appear more normal, or a dead person appear more lifelike, similar to the effect of red colorants in embalming fluid. The "false" or unphysiologic red-coloring effect in anoxic CO-poisoned tissue is related to the meat-coloring commercial use of carbon monoxide, discussed below.
Carbon monoxide also binds to other molecules such as myoglobin and mitochondrial cytochrome oxidase. Exposures to carbon monoxide may cause significant damage to the heart and central nervous system, especially to the globus pallidus, often with long-term chronic pathological conditions. Carbon monoxide may have severe adverse effects on the fetus of a pregnant woman.
[1] Hydrogen sulfide
[2] Carbon monoxide
The units can be managed via the dashboard. The data can be viewed remotely on a computer or on a mobile device.
| Item | Parameter | Ratings |
| Voltage | External | 5V micro-USB |
| Consumption | Current | 80mA |
| Connectivity | Internet | Wifi |
| Microcontroller | ESP8266 | 8 bit |
| Size and weight | Rainproof plastic | 57x44 (64 with brackets)x 60mm and 125grams |
Quick Setup
Connect it to power using a 5V micro-USB cable. Use a smartphone or a computer with WLAN capabilities to connect to the local hotspot spawned by your SENSIGAS unit. The SSID is uRADMonitor-XX, where XX are the last two digits of the Device ID number. The key is the Device ID, in uppercase, as printed on the enclosure. You can change this key later. Open 192.168.4.1 in your browser, and click the "WIFI/CONFIG" link to setup the connection to the Internet AP. Select the SSID and enter the key of your Internet Access Point.

USB configuration
Alternatively, the SSID and KEY can also be configured via USB. Connect to your SENSIGAS via USB, baudrate 9600bps, open a terminal program and type the two commands: "key1","SSID" then "key2","your WLAN key" . Use the "getsettings" command to verify that the new settings are in place. Quotes are a must, and there are no spaces in between. See the USB Commands manual for more. See the USB Commands manual if you need more help.
Technical Datasheet
USB Serial Commands Manual
Product Limited Warranty terms
uRADMonitor with WIFI Configuration manual
Open source on Github
Data access:
Terms of Service
API Terms of Service
API and Server Specs
Direct Data access
USB Driver
In their recent versions, Windows, Linux and MacOS come with built in drivers for your uRADMonitor unit. Use the following resources only if the built in drivers do not work for you.
USB Driver for Windows
USB Driver for Linux
USB Driver for MacOS
RO:
RO: Ghid rapid de utilizare
RO: conditii generale de utilizare
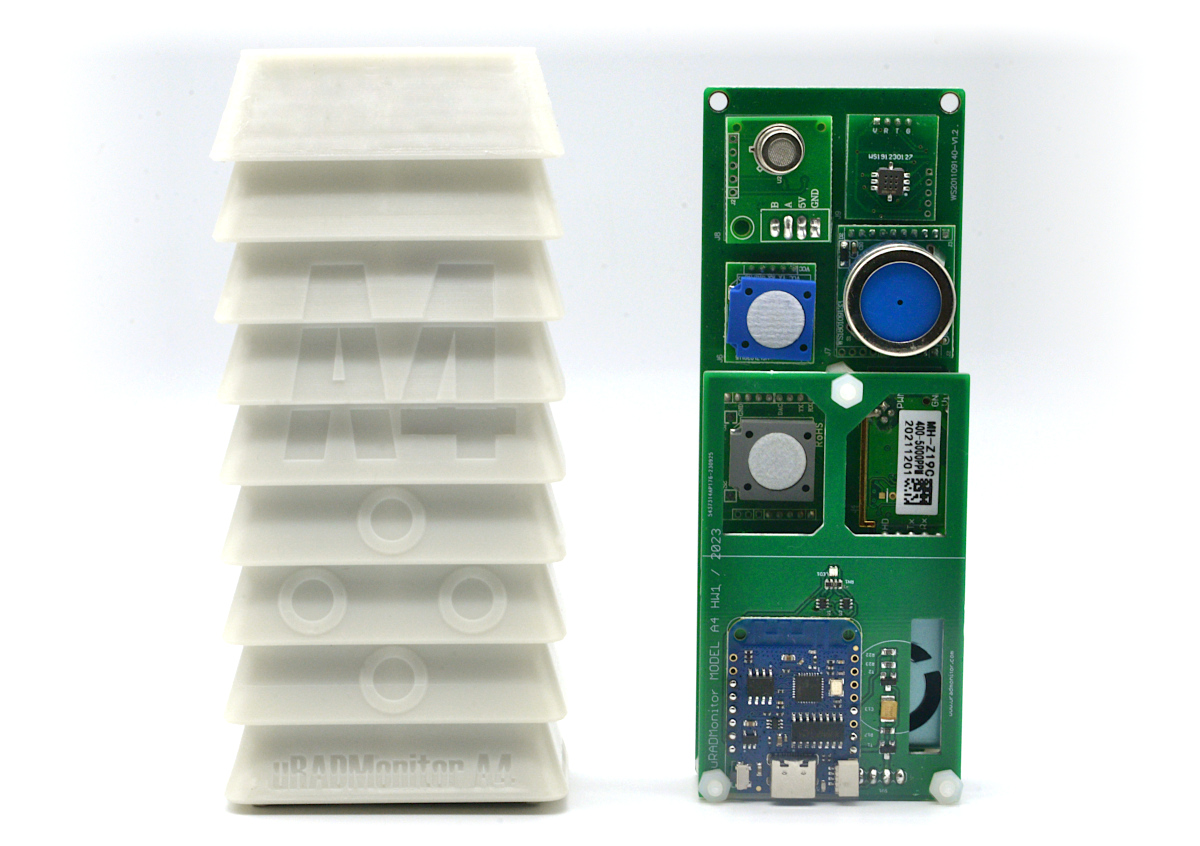
MODEL A4Contact us
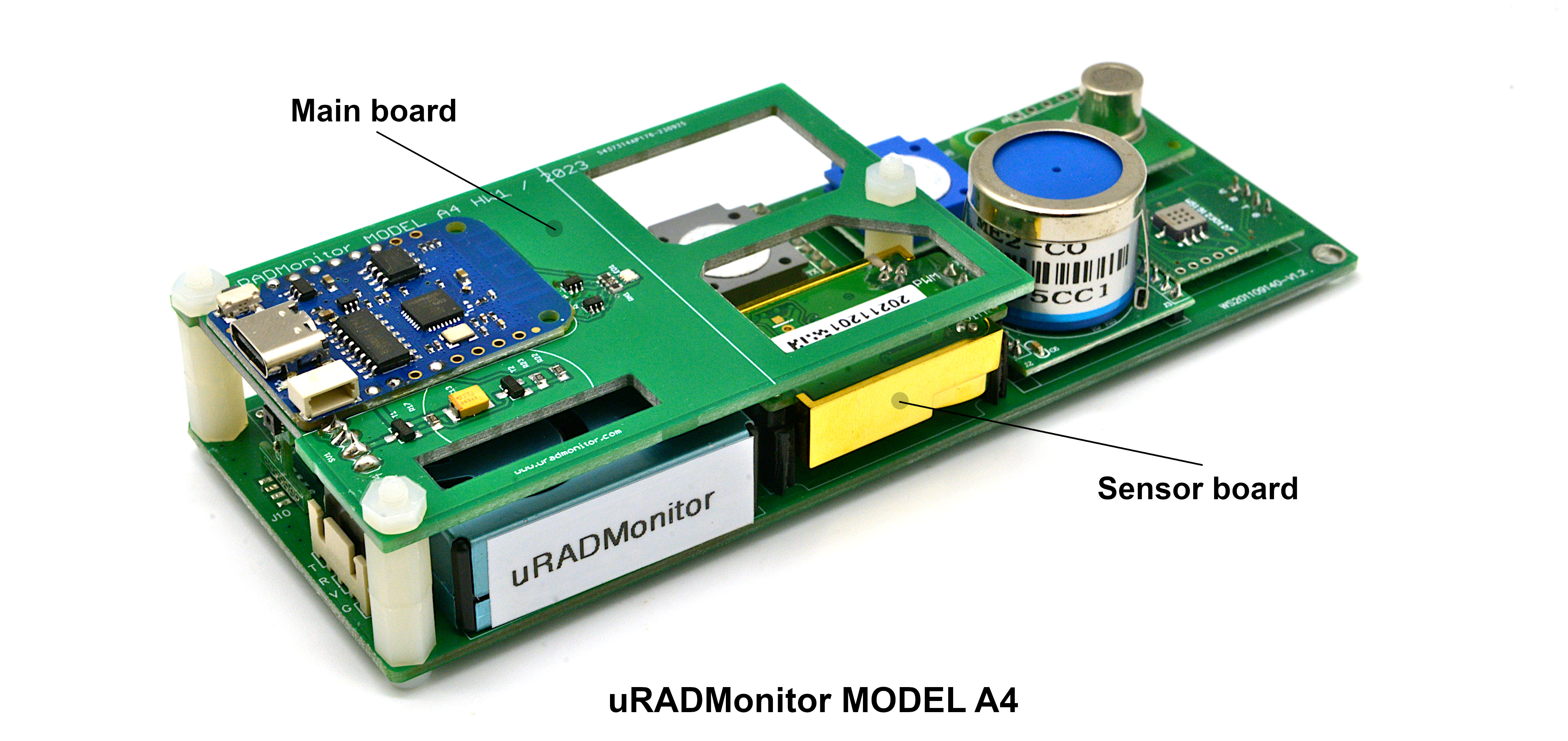
Multiparameter advanced air quality monitoring station, in a rainproof enclosure for both outdoor and indoor use. Contains 8 individual sensors to track Temperature, Relative Humidity, Nitrogen Dioxide, Formaldehyde, Carbon Monoxide, Ozone, Carbon Dioxide, Particulate Matter PM1, PM2.5, PM10 and Volatile Organic Compounds (VOC). Modular design that saves costs. Stevenson shield included, ready for outdoor use!
Chris, Australia: "Thanks guys, great project."
| Sensor | Parameter | Minimum value | Maximum value |
| MEMs | Temperature | -20 °C | +65 °C |
| Humidity | 0% RH | 100% RH | |
| Laser scattering | PM1.0 | 0 μg/m³ | 1000 μg/m³ |
| PM2.5 | 0 μg/m³ | 1000 μg/m³ | |
| PM10 | 0 μg/m³ | 1000 μg/m³ | |
| MEMs | Nitrogen Dioxide | 0 ppm | 10 ppm |
| Electrochemical | Formaldehyde | 0 ppm | 5 ppm |
| Electrochemical | Ozone | 0 ppm | 10 ppm |
| Electrochemical | Carbon Monoxide | 0 ppm | 500 ppm |
| NDIR | Carbon Dioxide | 400 ppm | 5000 ppm |
| MOX | VOC | 0 | 3 * |
Model A4 is a cost effective multiparameter automated monitor containing 8 high quality digital sensors that track a total of 11 air parameters. It has the capacity to fully characterize the ambient air in regards to health impact based on the major widespread pollutants.
Connect the uRADMonitor to the power source using the USB-C cable and the provided USB 5V Adaper.
uRADMonitor Model A4 Works via WIFI
Use a smartphone or a computer with WLAN capabilities to connect to the local hotspot spawned by your A4 unit. The SSID is uRADMonitor-XX, where XX are the last two digits of the Device ID number. The key is the "uradmonitor", in lowercase and without the quotes. You can change this default password later on. Open 192.168.4.1 in your browser, and click the "CONFIG" link to setup the connection to the Internet AP. Enter the SSID and key of your Internet Access Point. If the connection fails, you will see the status message, and three consecutive beeps will indicated the problem. Alternatively you can change the configuration using the USB data port. See the USB manual.
Remember, the Wifi settings can be customized via the USB connection. Consult your product manual for complete details.
Technical Datasheet
USB Serial Commands Manual
LoRaWAN Data Server Callback manual
Product Limited Warranty terms
uRADMonitor with WIFI Configuration manual
Data access:
Terms of Service
API Terms of Service
API and Server Specs
Direct Data access
USB Driver
In their recent versions, Windows, Linux and MacOS come with built in drivers for your uRADMonitor unit. Use the following resources only if the built in drivers do not work for you.
USB Driver for Windows
USB Driver for Linux
USB Driver for MacOS
RO:
RO: Ghid rapid de utilizare

 Available, ships right away.
Available, ships right away.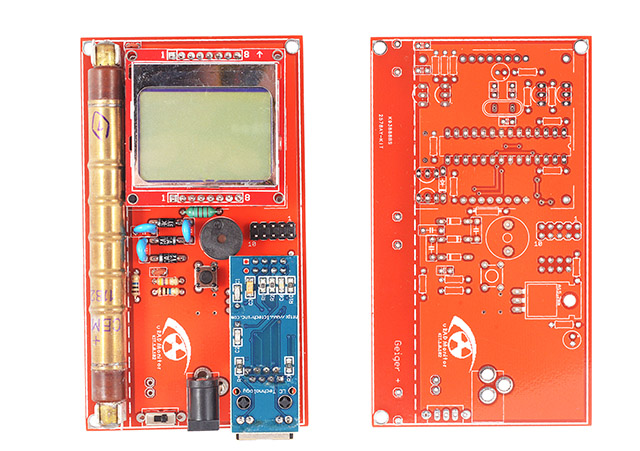
 Out of stock.
Out of stock.